Plasma membrane changes
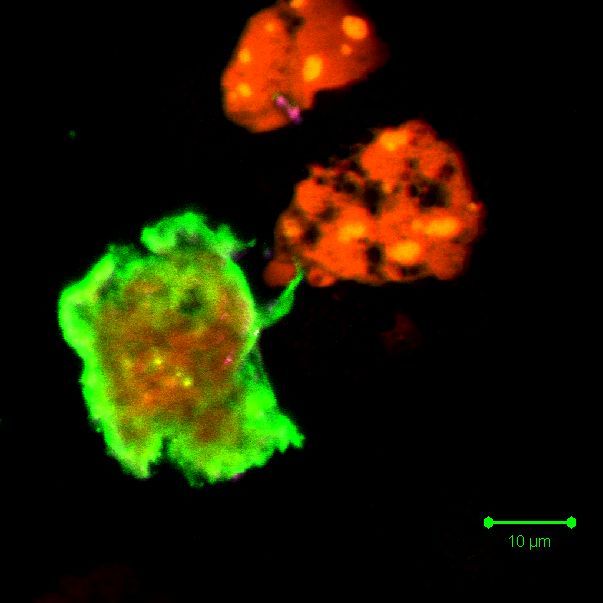
In normal cells, phosphatidylserine (PS) and phosphatidyl-ethanolamine (PE) residues are found in the inner membrane of the cytoplasmic membrane. During apoptosis, the PS and PE residues are translocated in the membrane and are externalized resulting in a change in surface charge of the outer leaflet of the plasma membrane. In general, though not always, this is an early event in oncosis and is thought to be a signal to neighbouring cells that a cell is ready to be phagocytosed. Annexin-V is a specific PS-binding protein that can be used to detect apoptotic cells by binding to PS.
Violet ratiometric asymmetry probe
The voltage change associated with the externalization of PS and PE can be detected by plasma membrane probe Bis-oxonol DiBAC4(5) and a new violet ratiometric membrane asymmetry probe, 4'-N,N-diethylamino-6-(N,N,N-dodecyl-methylamino-sulfopropyl)-methyl-3-hydroxyflavone or F2N12S is sensitive to changes in the potential difference or charge on the outer surface of the plasma membrane. The dye has an excited-state of intramolecular proton transfer or ESIPT reaction and in response to changes in the surface charge of the plasma membrane of cells emits fluorescence at two emissions of 530 and 585nm or green and orange fluorescence after excitation with a violet 405nm laser diode. Ratiometric analysis of the 2 emissions with the PMTs switched to linear gives all the advantages of ratiometric probes over single colour probes in that detected changes are independent of probe concentration, cell size and instrument variation in laser outputs and sensitivity of PMT detectors.
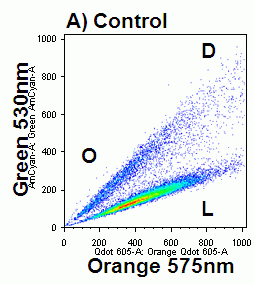
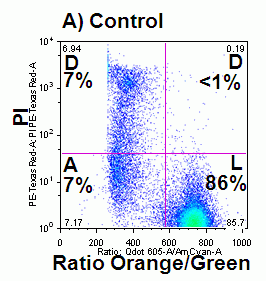
In apoptosis the surface charge of the outer plasma membrane changes which F2N12S can measure by alterations in the intensity of the two emissions, which emit more at 530nm (green) and less at 585nm (orange). Thus ratioing the 585nm emission by the 530nm emission electronically and plotting this Ratio parameter against cell viability shows the presence of apoptotic cells by a reduced Ratio signal. Dead cells also have a reduced Ratio signal compared to healthy live cells, see figure.
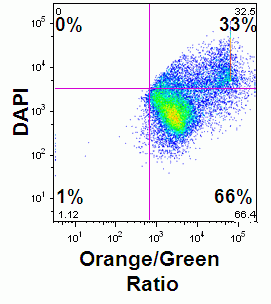
The F2N12S violet ratiometric membrane asymmetry probe is loaded into cells at RT for 5 minutes at 100nM concentration. Cells are not washed after this reaction but must be read within 30 minutes of loading cells. Then annexin V needs to be labeled before F2N12S, if a comparison of the two methods is required. Also although the emission spectra from F2N12S is at 530 and 575nm after 405nm excitation, DAPI with an emission spectra of 461nm cannot be used as a cell viability marker as there appears to be quenching of signals, see figure.
In addition other biological events can also be measured simultaneously such as mitochondrial membrane potential and Reactive Oxygen Species (ROS) dyes can also be loaded first at 37C, see figure.
Bis-oxonol DiBAC4(5) plasma membrane probe
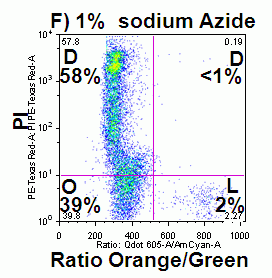
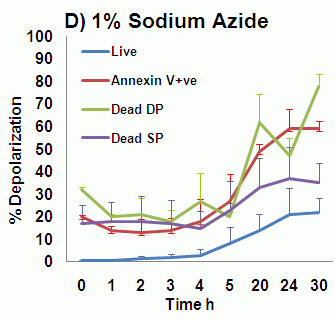
The rapid changes in plasma membrane potential observed during the initial stages of oncosis can also be measured in a time dependent manner over a 30 hour period in a similar manner to that of annexin V binding, see figure. A higher proportion of cells undergoing oncosis show depolarization of the plasma membrane, especially cells that are dead and bind annexin V; as does annexin V binding cells, dead cells that do not bind annexin V and even live cells although these do maintain membrane integrity they have little mitochondrial function, see figure. See also the section Viability on the role of plasma membrane depolarization in phases of cell death.
In addition other biological events can also be measured simultaneously such as mitochondrial membrane potential and Reactive Oxygen Species (ROS) dyes can also be loaded first at 37C.
Numerous DNA binding viability dyes can be used with the violet ratiometric asymmetry or bis-oxonol probes including PI, 7-AAD, SYTOX AADvanced, and DRAQ7 (Biostatus).
- Model of plasma membrane
- Violet membrane asymmetry probe ratio
- Violet membrane asymmetry probe and oncosis
- Violet membrane asymmetry probe and mitochondrial function
- Time-dependent changes in plasma membrane potential during oncosis
- Protocol for F2N12S probe