ER Stress
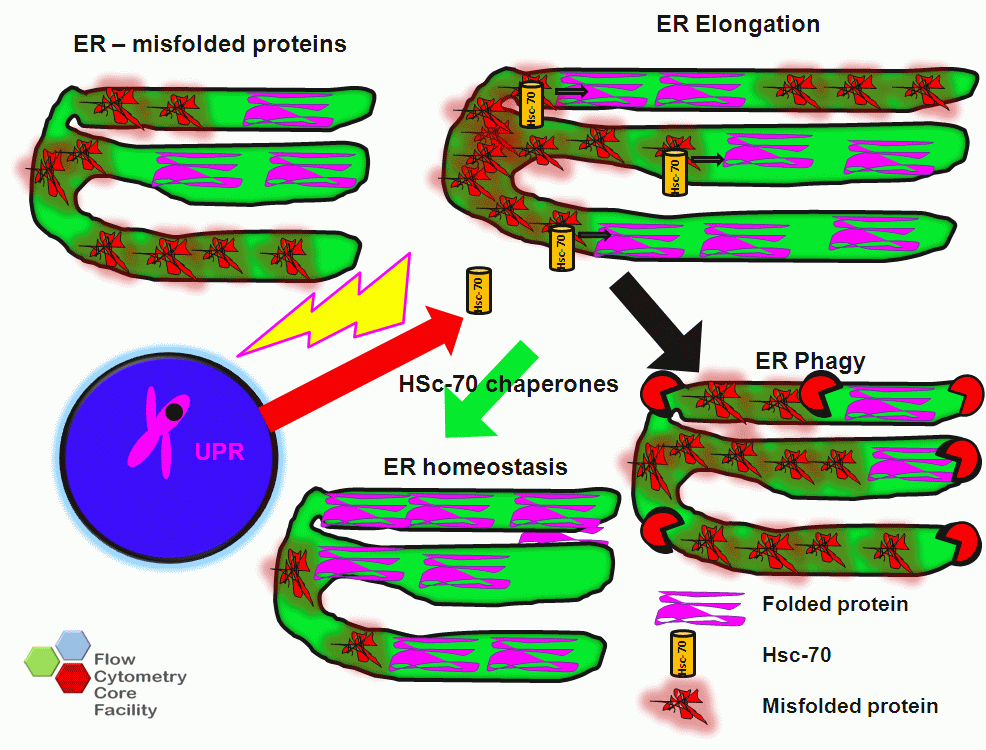
The ER is responsible for the folding and then delivery of proteins via the secretory pathway to a functional site. Misfolded proteins accumulate in the lumen of the ER due to high protein folding demand on the ER especially during autophagy. Only properly folded proteins are secreted with maintenance of the plasma membrane structure and ER folding capacity being under ER homeostatic control. Once a threshold of mis-folded protein accumulation has been reached, a signal activates the ER to nucleus signalling pathway or the unfolded protein response (UPR) causing ER chaperone proteins to be synthesised, which refold the mis folded proteins and translocate these proteins to the cytoplasm for degradation by the proteasome. This process results in an increase of the ER capacity to fold proteins and maintain ER homeostasis. This increase in ER capacity is physically achieved by an increase in size of the ER at early stages of autophagy, see figure.
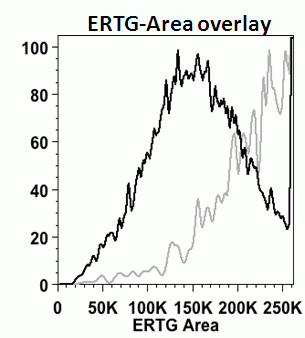
If the protein folding demand continues to increase, this will ultimately result in the phagy of the ER itself, which can be caused by ER stress as induced in vitro by thapsigargin, chloroquine (CQ), tunicamycin and dithiothreitol, DT), see figure.
Schematic of Reticulophagy
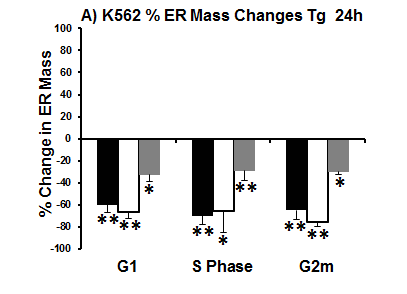
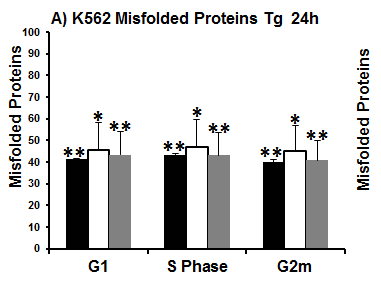
However in normal Jurkat T-cells & K562 cells showed increasing ER mass as the cell moves through the cell cycle, with S phase cells showed a 36% & 27% increase in ER mass above that detected in cells in G1 respectively. While cells in G2m show a 16% & 18 % increase in ER mass above that of S phase cells respectively, see figure.
Aggresomes
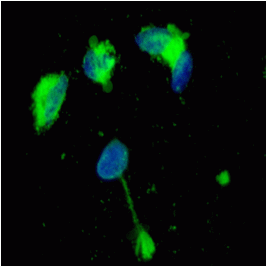
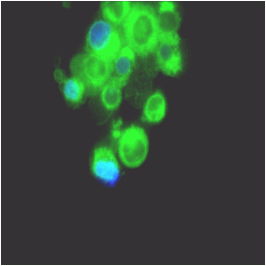
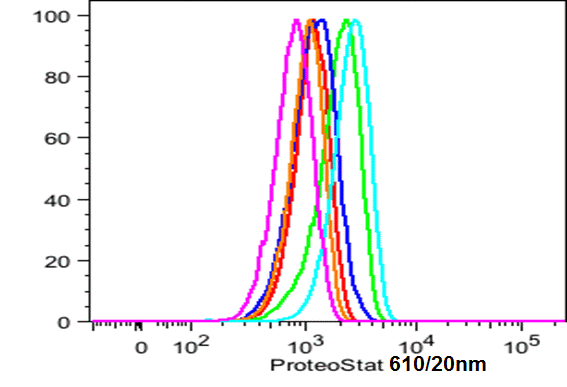
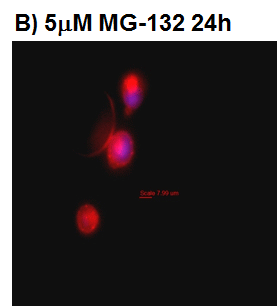
prior to macroautophagy induced ER phagy and the accumulation of mis-folded proteins within the ER, inclusion bodies form when the ubiquitin-proteasome machinery is overwhelmed by cytostolic protein aggregates to form transient structures termed Aggresome-like Induced structures or ALIS. The formation of ALIS has been shown to require signalling via mTOR and is thus independent of autophagy with ALIS being cleared by lysosomes alone. The binding of LPS or pathogens to TLR receptors not only causes NF-KB acitvation with mTOR signalling but up-regulation of p62 to form ALIS. Aggresomal responses are thought togive rise to Lewy bodies anf hyaline inclusion bodies observed in amyotrophic lateral sclerosis (ALS). Aggresosmes can be detected by use of the Proteostat Aggresome detection kit from Enzo Life Sciences which uses a red fluorescent molecular rotor dye which specifically detects denatured protein cargo within aggresomes and ALISs. Flow cytometry can used as well as fluorescent microscopy by excitation with blue laser and collection at 610nm. Protease inhibitor MG-132 can be used as a positive control for aggresome formation, see figure. For the link to Enzo Life Sciences Website for data supplied from this Flow Cytometry Core Facility
Cells producing misfolded proteins fthrough ER stress can also be measured in a cell cycle dependent manner, see figure.